Answer:
C) 43,2°C
Step-by-step explanation:
Sensible heat is the amount of thermal energy that is required to change the temperature of an object, the equation for calculating the heat change is given by:
Q=msΔT
where:
- Q, heat that has been absorbed or realeased by the substance [J]
- m, mass of the substance [g]
- s, specific heat capacity [J/g°C]
- ΔT, changes in the substance temperature [°C]
To solve the problem, we clear ΔT of the equation and then replace our data:
Q=890 [J],
m=16,6 [g],
s=2,74 [J/g°C]
Q=msΔT.......................ΔT=Q/ms
Δ
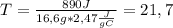 °C
°C
As:
ΔT=Tfinal-Tinitial
Tfinal=ΔT+Tinitial
Tfinal=21,7+21,5=43,2°C
The final temperature of the ethanol is 43,2°C.