Answer:
The property helps to explain this difference is forces between molecules.
Option c
Step-by-step explanation:
The exact heat of water is
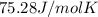
The exact heat of methane is
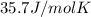
This is due to the polarity and the hydrogen bonding.
It takes more energy to break these bonds than methane, therefore, we would think water to have a higher specific heat due to these active forces.
So this property helps to explain there is differences in forces between molecules.