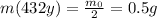Answer:
0.5 g
Step-by-step explanation:
The half-life of a radioactive isotope is the time it takes for a certain amount of that isotope to halve.
In this case, we have an isotope of Americium-241, which has a half-life of
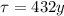
This means that after 1 half-life (432 years), there will be an amount of americium equal to half its initial amount.
In this problem, the initial amount of americium is
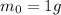
Therefore, after 432 years (1 half-life), the amount of americium left will be half: