Step-by-step explanation:
Energy levels of a hypothetical atom:
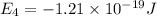
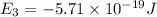
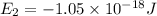
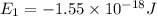
a) Wavelength of the photon needed to excite an electron from
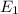 to
to
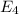 .
.
Energy difference between forth and first energy level =
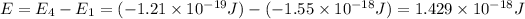
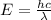
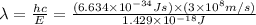
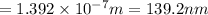
139.2 nm is the wavelength of the photon needed to excite an electron from
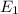 to
to
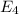 .
.
b) Energy of a photon in order to excite an electron from
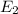 to
to
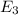 .
.
Energy difference between third and second energy level =
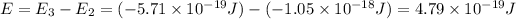
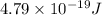 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from
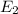 to
to
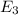 .
.
c) Electron drops from the
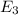 level to the
level to the
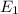 level
level
Energy difference between third and first energy level =
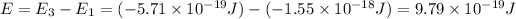
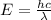
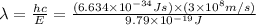
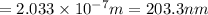
202.3 nm is the wavelength of the photon of emitted.