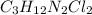Answer:
Step-by-step explanation:
Lets say the formula of the compound is:
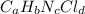
Experiment 1:
With 1.00 g of the compound you get 1.95 g of AgCl
The molar mass of AgCl is 143.32 g/mol.
The moles of AgCl in 1.95g are:
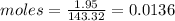
Also the moles of Cl are 0.0136 because 1 mol of AgCl has 1 mol of Cl
Experiment 2:
With 1.00 g of the compound you get 0.900 of CO2 and 0.735 g of H2O
The molar mass of CO2 is 44 g/mol
The molar mass of H2O is 18 g/mol
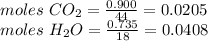
moles of C are 0.0205
moles of H are 0.0816 (2 times the moles of H2O)
With the given information so far you get that in 1.00 g of the compound there are 0.0136 moles of Cl, 0.0205 moles of C and 0.0816 moles of H.
In mass you have:
Mass Cl = 0.0136 * 35.5 = 0.4828 g
Mass C = 0.0205 * 12 = 0.246 g
Mass H = 0.0816 * 1 = 0.0816 g
Total mass = 0.4828 + 0.246 + 0.0816 + mass N
1.00 = 0.8104 + Mass N
Mass N = 0.1896
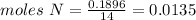
Now you divide all moles for the least
Cl: 0.0136 / 0.0135 = 1.0007
C: 0.0205 / 0.0135 = 1.52
H: 0.0816 / 0.0135 = 6.04
N: 0.0135 / 0.0135 = 1
multiplying by 2 to get an integer for C
Cl = 2
C = 3
H = 12
N = 2