Answer:
The equilibrium constant of the reaction at this temperature is

Step-by-step explanation:
Equilibrium is the condition at which the reactants and products concentration is constant.
At equilibrium rate of the forward reaction = rate of the backward or reverse reaction
The Chemical equilibrium is
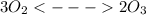
 is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the
is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the
 expressed as
expressed as
![K_c = \frac {[Products concentration]}{[Reactants concentration]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/buyw0trep9pnibmquff3bnjptfzviqedzy.png)
Plugging in the values given in the question, we have
![K_c =\frac {[O_3 ]^2}{[O_2 ]^3}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/7r4vth3l2auriv8v1c0nbt21amhvvuyu9r.png)
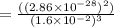
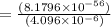
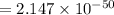 (Answer)
(Answer)