Answer: C) 80 L
Explanation:
According to the dilution law,
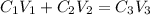
where,
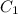 = concentration of acid solution = 50 %
= concentration of acid solution = 50 %
 = volume of acid solution = 40 L
= volume of acid solution = 40 L
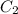 = concentration of another acid solution= 20%
= concentration of another acid solution= 20%
 = volume of another acid solution= x L
= volume of another acid solution= x L
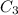 = concentration of resulting acid solution = 30 %
= concentration of resulting acid solution = 30 %
 = volume of resulting acid solution = (40+x) L
= volume of resulting acid solution = (40+x) L
Putting the values in the equation:

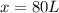
Thus 80 L of the 20% acidic solution should be added to create the needed 30% acidity in the final mixture.