Step-by-step explanation:
The given data is as follows.
Mass of Mg = 1.30 g, Molarity = 5 for HCl, Volume = 150 mL
Density = 1.1 g/mL
Change in temperature (dT) =
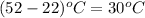
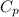 = 345
= 345
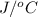
Hence, the energy balance will be as follows.
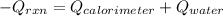
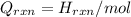
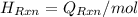
Moles of Mg =
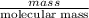
=
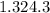
= 0.05349 mol of Mg
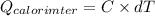
=
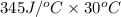
= 10350 J
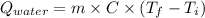
=
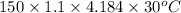
= 20710.8
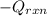 = (20710.8 + 10350) J
= (20710.8 + 10350) J
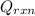 = -31060.8 J
= -31060.8 J
Therefore, calculate the value of
 of the reaction as follows.
of the reaction as follows.
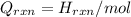
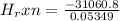
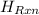 = -580684.24 J/mol
= -580684.24 J/mol
or,
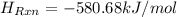 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)
Thus, we can conclude that value of
 of the reaction under given conditions is -580.68 kJ/mol.
of the reaction under given conditions is -580.68 kJ/mol.