Answer:
The empirical formula of cyclohexene is C₃H₅
Step-by-step explanation:
Given parameters:
Mass of CO₂ produced = 4.822g
Mass of H₂O produced = 1.650g
Unknown:
Simplest formula of cyclohexene or empirical formula of cyclohexene = ?
Solution:
We first write the balanced combustion equation of the cyclohexene in oxygen gas:
Cyclohexene = C₆H₁₀
2C₆H₁₀ + 17O₂ →12CO₂ + 10H₂O
From the gases produced, we can obtain the masses of the constituent atoms that makes up cyclohexene. Cyclohexene which is a hydrocarbon is made up of hydrogen and carbon atoms:
Mass of C in CO₂ =
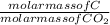 x mass of CO₂
x mass of CO₂
Mass of C =
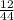 x 4.822 = 1.3151g
x 4.822 = 1.3151g
Mass of H in H₂O =
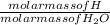 x mass of H₂O
x mass of H₂O
Mass of H =
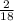 x 1.650 = 0.1833g
x 1.650 = 0.1833g
Using these masses, we can find the empirical formula of the hydrocarbon:
C H
Masses(g) 1.3151 0.1833
Number
of moles
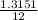
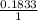
0.1096 0.1833
Simplest
form
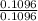
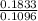
1 1.672
Empirical formula is usually expressed to the simplest whole number ratios. Therefore, to obtain the simplest whole number ratio here, simply multiply by a factor of 3:
1 x 3 1.672 x 3
3 5
The empirical formula of cyclohexene is C₃H₅