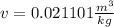Answer:
a. 0.01075866 m^3/kg
b.0.021101 m^3/kg
Step-by-step explanation:
a. From thermodynamic tables we get the value for specific volume for R134a in the condition of saturation. We get the value for saturated vapor (v_g) and saturated liquid (v_f) at 45°C. These are:
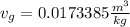
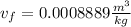
The specific volume as a function of the quality in the saturation region is defined as:
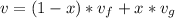
So, for a quality of 0.6 we have:
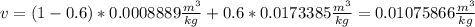
b. For a pressure of 1Mpa at 45°C
The pressure of saturation at 45°C is 1.16 MPa since the pressure in this condition (1MPa) is lower than the pressure of saturation we have superheated R134a.
Checking the thermodynamic tables for superheated R134a we get the value for specific volume at this condition.
This is: