Answer:
final pressure = 0.7 MPa
quality = 20 degree super heat.
Step-by-step explanation:
Given data:
water temperature 120 degree celcius
quality
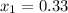
we know that from saturated water temperature table . for T =120 DEGREE
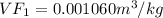
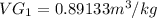
we know that
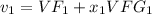
= 0.001060 + 0.33(0.89133- 0.001060)
= 0.29485 m^3/kg
frinal temperature of water is
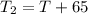
= 120 + 65 = 185 Degree
from saturated water temp. table
for T = 185 degree, VG = 0.17390 m^3/kg
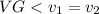
from super heated table by interpolation
final pressure = 0.7 MPa
quality = 20 degree super heat.