Answer:
W = - 523.425 W = -0.5234 kW
Negative sign show power input to the pump
Step-by-step explanation:
By using energy balanced at state q and state 2
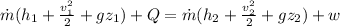
As it is given neglect kinetic energy and heat transfer therefore above equation rduece to
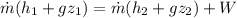
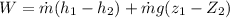
As temp remain cosntant , so enthalapy difference is givena s
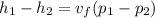
from saturated water tables, for temperature 15 degree celcius specific volume of water is
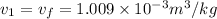
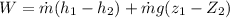
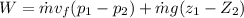
putting zi =0, z2 = 15, m= 1.5 kg/s
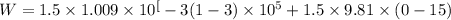
W = - 523.425 W
Negative sign show power input to the pump