Answer:
K is more reactive.
Step-by-step explanation:
We follow the reactivity series to say which one is more reactive than the other.
Oxidation is loss of electrons by an atom or a species.
The element which gets oxidised easily is more reactive than the one which is less oxidised.
Zn undergoes more easily than Copper. Thus we say Zinc is more reactive than Copper.
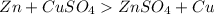
Zinc is more reactive than copper thus it replaces copper in this reaction.
Copper cannot replace Zinc as it is less reactive. Thus No reaction occurs when Copper reacts with Zinc sulphate
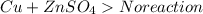
We can observe this in the reactivity series or electrochemical series which is as follows
more reactive ……………………………………………………………………………….
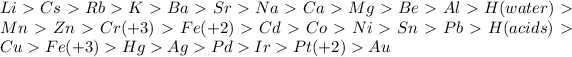
…………………………………………………………………………………..least reactive
So, The Answer will be K and Ca.
Moving down a group, the reactivity decreases.
Hence we can say that K is more reactive than Mg.