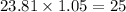Answer:
when 5% excess air is supplied, moles of air supplied/moles of fuel =
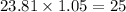
Step-by-step explanation:
Equivalence ratio = 0.6
Equivalence ratio = Actual air to fuel ratio (AAFR)/ stoichiometric air to fuel ratio SAFR
combustion reaction of propane is
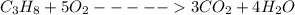
From above reaction, 1 mole of propane, from the reaction, 5 moles of oxygen required,
we know that air contains 21% O_2 and 79% N_2,
Therefore, moles of air based on stoichiometry
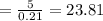
Theoretical air to fuel ratio
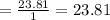
Given
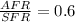
Actual Air Fuel Ratio
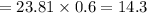
when 5% excess air is supplied, moles of air supplied/moles of fuel =