Answer:
Work input =283.47 KJ
Step-by-step explanation:
Given that
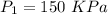
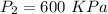
T=12°C=285 K
m= 2.5 kg
Given that this is the constant temperature process.
e know that work for isothermal process
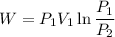
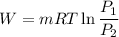
So now putting the values
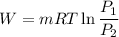
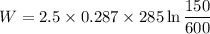
W=-283.47 KJ
Negative sign indicates that work is done on the system.
So work input =283.47 KJ