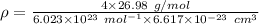Answer:
Edge length = 0.40446 nm =
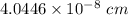
Volume = 0.06617 nm³ =
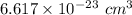

Step-by-step explanation:
Given that:
The radius = 0.143 nm
For FCC,
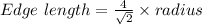
Thus,
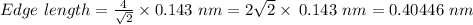
Edge length = 0.40446 nm
Also, 1 nm =
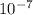 cm
cm
So,
Edge length =
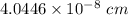
Volume =
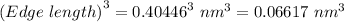
Volume = 0.06617 nm³
Also, 1 nm³ =
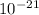 cm³
cm³
Volume =
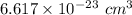
The expression for density is:
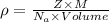

M is molar mass = 26.98 g/mol
For FCC , Z= 4
Thus,