Answer:
The alcohol concentration of the resulting solution is 58.4%
Explanation:
- At first we must to find the quantity of pure alcohol in the 8 liters
∵ The alcohol concentration is 48% in 8 liters
∴ The quantity of pure alcoholic =
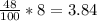 liters
liters
- She added 2 liters pure alcohol to the solution that means the solution
increased by 2 liters and alcohol quantity increased by 2 liters
∵ She added 2 liters pure alcohol
∴ The quantity of pure alcohol = 2 + 3.84 = 5.84 liters
∴ The resulting solution = 2 + 8 = 10 liters
- Now we need to find the concentration of alcohol in the resulting
solution
∵ The quantity of pure alcohol = 5.84 liters
∵ The resulting solution = 10 liters
∴ The concentrate of alcohol =
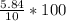 % = 58.4%
% = 58.4%
The alcohol concentration of the resulting solution is 58.4%