Answer:
|W|=169.28 KJ/kg
ΔS = -0.544 KJ/Kg.K
Step-by-step explanation:
Given that
T= 100°F
We know that
1 °F = 255.92 K
100°F = 310 .92 K
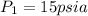
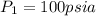
We know that work for isothermal process
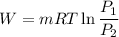
Lets take mass is 1 kg.
So work per unit mass
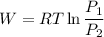
We know that for air R=0.287KJ/kg.K
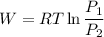
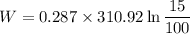
W= - 169.28 KJ/kg
Negative sign indicates compression
|W|=169.28 KJ/kg
We know that change in entropy at constant volume
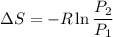
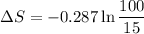
ΔS = -0.544 KJ/Kg.K