Answer:
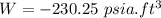
Step-by-step explanation:
Given that
p V = 100
This is the isothermal process.
We know that non-flow work given as
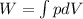
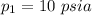
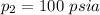
We know that work for isothermal process
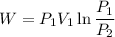
For isothermal process
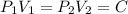
Here C= 100
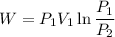
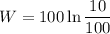
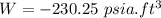
Negative sign indicates that work in done on the system.