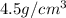Answer : The correct option is, (3)
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used :

First we have to calculate the volume of cuboid.
Formula used :

where,
V = volume of cuboid
l = length of cuboid = 6.0 cm
b = breadth of cuboid = 3.0 cm
h = height of cuboid = 2.0 cm
Now put all the given values in the above formula, we get:
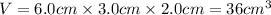
Now we have to calculate the density of cube.
Given :
Mass of cube = 162.2 g
Volume of cube =
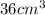
Now put all the given values in the above formula, we get :
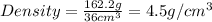
Therefore, the density of cube is