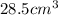Answer : The volume of a sample of 4.00 mol of copper is
Explanation :
First we have to calculate the mass of copper.
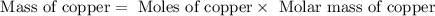
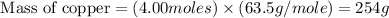
Now we have to calculate the volume of copper.
Formula used :

Now put all the given values in this formula, we get:
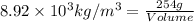
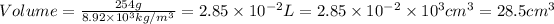
Conversion used :
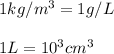
Therefore, the volume of a sample of 4.00 mol of copper is