Answer:
Density of water decreases when it becomes ice.
Step-by-step explanation:
Density of a quantity that relates a mass to the volume it occupies
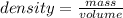 , this tells us that there is an inverse relationship between density and volume: as the volume that a mass occupies increases, its density decreases.
, this tells us that there is an inverse relationship between density and volume: as the volume that a mass occupies increases, its density decreases.
The density of water when moving from a liquid state to a solid (frozen) state decreases, because solid water occupies a larger volume.
And because objects with a lower density than liquid water tend to float, this is why ice cubes float.