Answer:
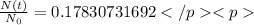
Step-by-step explanation:
There are many formulas that can describe the exponential decay of a substance. For example, one of the formulas we could use for the quantity that still remains after a time t, given an original quantity
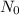 and a half-life of
and a half-life of
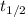 is:
is:
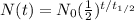
We want to calculate what fraction of the original shipment would still we have, that is,
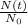
This is why it is useful to use the formula already written, now we can just calculate:
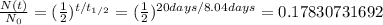 , which means that around 17.83% of the original substance has not decayed yet.
, which means that around 17.83% of the original substance has not decayed yet.