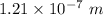Answer:
The frequency is
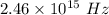
The wavelength is
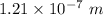
Step-by-step explanation:
Given that,
A photon emitted by a transition of an electron from a n-2 orbit to n-1 orbit.
We know that,
For hydrogen atom, energy emitted due to transition of electron between two states
We need to calculate the energy
Using formula of energy
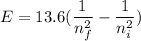
Put the value into the formula
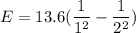
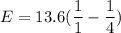
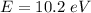
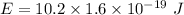
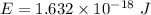
We need to calculate the frequency
Using formula of frequency
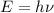
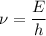
Put the value into the formula
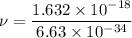
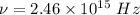
We need to calculate the wavelength
Using formula of wavelength
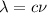
Put the value into the formula
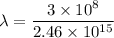
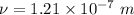
Hence, The frequency is
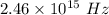
The wavelength is