Answer:
1533.6 kg NO
Step-by-step explanation:
The reaction that takes place is:
First we convert the masses of ammonia (NH₃) and oxygen gas (O₂) into moles, using their respective molar masses:
- NH₃ ⇒ 869 kg ÷ 17 kg/kmol = 51.12 kmol NH₃
- O₂ ⇒ 2480 kg ÷ 32 kg/kmol = 77.5 kmol O₂
77.5 kmol of O₂ would react completely with (77.5 kmol O₂ *
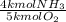 ) 62 kmol of NH₃. There are not as many kmol of NH₃, so NH₃ is the limiting reactant.
) 62 kmol of NH₃. There are not as many kmol of NH₃, so NH₃ is the limiting reactant.
Now we calculate how many kmol of NO are produced, using the limiting reactant moles:
- 51.12 kmol NH₃ *
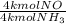 = 51.12 kmol NO
= 51.12 kmol NO
Finally we convert kmol of NO to mass, using its molar mass:
- 51.12 kmol NO * 30 kg/kmol = 1533.6 kg NO