Answer:
The kinetic energy is 86.6 zepto joules.
Step-by-step explanation:
Given that,
Number of orbit n =5
We know that,
Bohr's radius for hydrogen atom is
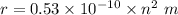
Now, put the value of n in the formula of radius
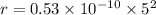
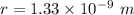
We need to calculate the kinetic energy
Using formula of kinetic energy
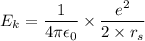
Put the value into the formula
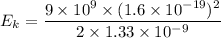
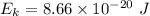
We know that,
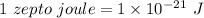
The kinetic energy is
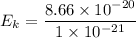
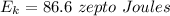
Hence, The kinetic energy is 86.6 zepto joules.