Answer:
Number of protons =
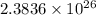
Number of electrons =
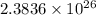
Charge on these electrons = -

Step-by-step explanation:
1 atom of lead contains 82 protons and 82 electrons
1 mole =
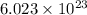 atoms
atoms
Thus,
1 mole of lead contains
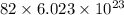 protons
protons
Also, 1 mole of lead weighs 207.2 g
So,
207.2 g of lead contains
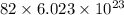 protons
protons
1 kg = 1000 g
So,
1000 g or 1 kg of lead contains
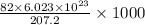 protons
protons
Number of protons =
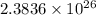
Also, for neutral atom, number of protons = number of electrons.
Thus,
Number of electrons =
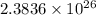
Also,
Charge of 1 electron = -
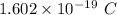
Charge on these electrons = -

Charge on these electrons = -
