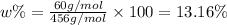Answer:
The weight% of
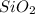 in augite is 13.16 %.
in augite is 13.16 %.
Step-by-step explanation:
Atomic mass of calcium = 40 g/mol
Atomic mass of iron = 56 g/mol
Atomic mass of magnesium = 24 g/mol
Atomic mass of sodium = 23 g/mol
Atomic mass of aluminum = 27 g/mol
Atomic mass of silicon = 28 g/mol
Atomic mass of oxygen = 16 g/mol
Mass of
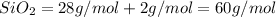
Molar mass of the augite
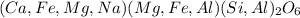 = M
= M
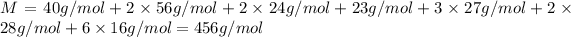
Weight% of
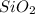 in augite:
in augite: