Answer : The value of
 is 4.76 J
is 4.76 J
Explanation :
Formula used :
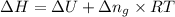
According to the ideal gas equation,
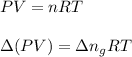
So,
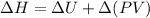
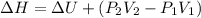
where,
 = internal energy of the reaction = -358 J
= internal energy of the reaction = -358 J
 = enthalpy of the reaction = ?
= enthalpy of the reaction = ?
 = initial pressure = 0.36 atm
= initial pressure = 0.36 atm
 = final pressure = 0.34 atm
= final pressure = 0.34 atm
 = initial volume = 8 L
= initial volume = 8 L
 = final volume = 19 L
= final volume = 19 L
Now put all the given values in the above formula, we get:
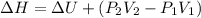
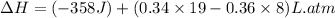
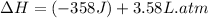
conversion used :
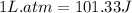
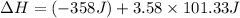
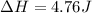
Therefore, the value of
 is 4.76 J
is 4.76 J