Answer:
Group 17 or VIIA which consists of Halogens have elements existing in solid, liquid and gas at room temperature (20 degree celcius).
Step-by-step explanation:
Fluorine, Chlorine, bromine, Iodine and Astatine are the halogens we know.
At room temperature,
Fluorine and chlorine are gases,
Bromine is a liquid and
Iodine and Astatine are solids.
Halogens are called as Salt producer.
They are diatomic elements except Astatine with a subscript 2 which represents a molecule of halogen is comprised of two halogen atoms
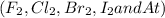
The general electronic configuration of halogen is
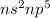
The elements present in the same group will have same number of valence electrons (outer shell electrons)
For example
Fluorine, Chlorine, Bromine are all present in the same Group
The electronic configurations are
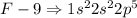
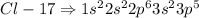
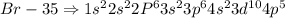
Hence all these elements have similar chemical properties since it depends on the electrons present in an element.