Answer:
The mass percent composition is 53.2852 %
Step-by-step explanation:
The formula used to calculate the % composition is
% composition = (mass of the element)/(total mass of the compound) × 100%
Total mass of the compound
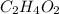
= (12.0107 × 2) + (1.0079 × 4) + (15.999 × 2)
= 24.02140 + 4.03176 + 31.99880
= 60.05196 g
Mass of the element, O = 31.99880 g
% composition of O
= (mass of the element) / (total mass of the compound) × 100%
Plugging into the formula
% composition of O
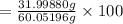
= 53.2852 % (Answer)