Answer:
11.7 g in 1 L of water.
Step-by-step explanation:
Molarity (M) or Molar concentration is a measure used in chemistry for solutes in a solution.
It is defined as mol/ L (number of moles per litre).
0,2 M means we have 0,2 mol/ L
In order to know how many grams this means we need to look up the molecular weight of the solute. In this case NaCl: 58.443 g/mol
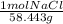 =
=
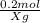
X=
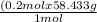
X= 11.69 g
The solution has 11.69 g of NaCl in 1 L of water since its an adequate polar solvent for NaCl dissolution.