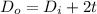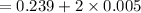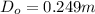Answer:
Step-by-step explanation:
Given data:
Mass of cylinder is 5 kg
Pressure = 200 atm
Temperature = 20 degree celcius = 293 K
wall thickness = 0.5 cm = 0.005 m
we know that Gas constant for nitrogen is calculated as
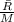
![R = (8314)/(28.01) = 296.82 J/kg.K</p><p>FROM IDEAL GAS EQUATION</p><p>PV =mRT</p><p>[tex]V = (mRT)/(P)](https://img.qammunity.org/2020/formulas/engineering/college/y7wkgedjvqpjzhhl4wtn0epqb9l7w8ov12.png)
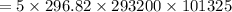
V = 0.021457 m^3
internal diameter by using volume formula
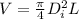
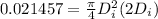
solving for
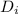
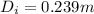
calculate the outer diameter