Answer:
r= 0.9949 (For 15,000)
r=0.995 (For 19,000)
Step-by-step explanation:
We know that
Molecular weight of hexamethylene diamine = 116.21 g/mol
Molecular weight of adipic acid = 146.14 g/mol
Molecular weight of water = 18.016 g/mol
As we know that when adipic acid and hexamethylene diamine react then nylon 6, 6 comes out as the final product and release 2 molecule of water.
So
![M_(repeat)=146.14+166.21-2* 18.106\ g/mol]()
![M_(repeat)=226.32\ g/mol]()
So
Mo= 226.32/2 =113.16 g/mol
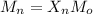
Given that
Mn= 15,000 g/mol
So
15,000 = Xn x 113.16
Xn = 132.55
Now by using Carothers equation we know that
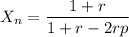
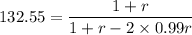
By calculating we get
r= 0.9949
For 19,000
19,000 = Xn x 113.16
Xn = 167.99
By calculating in same process given above we get
r=0.995