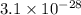Answer: The equilibrium constant for this reaction is
Step-by-step explanation:
To calculate the equilibrium constant (at 15°C) for given value of Gibbs free energy, we use the relation:
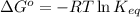
where,
 = standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
= standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
R = Gas constant =
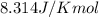
T = temperature =
![15^oC=[273+15]K=283K](https://img.qammunity.org/2020/formulas/chemistry/college/v2x730dssa1j9wlfa8kw7tqti3ju861f0s.png)
 = equilibrium constant at 10°C = ?
= equilibrium constant at 10°C = ?
Putting values in above equation, we get:
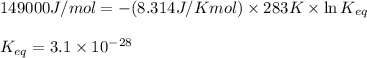
Hence, the equilibrium constant for this reaction is