Answer:
By adding 2.88 milliliter of water to the 0.12 milliliter of 0.25 M solution of sodium citrate will give 25-fold diluted solution.
Step-by-step explanation:
25 fold dilution means that the concentration of solution is reduced by one by twenty fifth times of the original solution.
Fold - dilution =
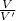
V =Volume of original solution before dilution
V' = Total Volume of solution after dilution
V = x
V'= 3
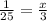
x =
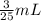
Total Volume of solution after dilution = V + y
y = Amount of solvent added ( here is water)
V + 3 = 3 mL
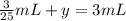
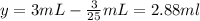
Volume of water added = 2.88 mL
Volume of original solution = 3 ml - 2.88 ml = 0.12 mL
By adding 2.88 milliliter of water to the 0.12 milliliter of 0.25 M solution of sodium citrate will give 25-fold diluted solution.