Answer:
By criss crossing the charge numbers we find the chemical formula of a compound
For example Aluminium chloride
Al has
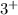 charge and Cl has
charge and Cl has
 charge
charge
3 becomes the subscript of Cl and 1 becomes the subscript of Al
So the chemical formula will be
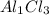
Ionic compounds are named using format as follows
Metal’s name + root word of the non metal + ide ending (when the metal has only one type of charge)
Example NaCl is named as Sodium + chlor + ide = Sodium chloride
Metal’s name + charge in roman numeral + root word of non metal + ide ending (when the metal possess more than one type of charge)
Example
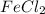 is named as Iron+(II)+chlor+ide =Iron(II)chloride
is named as Iron+(II)+chlor+ide =Iron(II)chloride
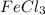 is named as Iron+(III)+chlor+ide =Iron(III)chloride
is named as Iron+(III)+chlor+ide =Iron(III)chloride
From the formula CuO we get to know the charge numbers of Cu is
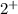 and O is
and O is
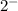
CuO is Copper (II) oxide
Metal + roman numeral for charge + root word of non metal + ide ending used in this
It is an ionic compound since it has a metal and a non metal.
When there is a polyatomic ion present for example
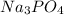 the name of the polyatomic ion is used as it is instead of ’ide’ ending
the name of the polyatomic ion is used as it is instead of ’ide’ ending
Thus
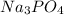 is Sodium phosphate
is Sodium phosphate
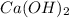
Ca possess only one type of charge that is 2+ .So we don’t use roman numeral for the metal.
 is Hydroxide ion and it is a polyatomic ion
is Hydroxide ion and it is a polyatomic ion
So the name of the compound is Calcium hydroxide