Step-by-step explanation:
The given data is as follows.
Molarity of
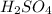 = 0.500 M
= 0.500 M
Volume
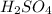 solution = 21.7 mL
solution = 21.7 mL
Hence, calculate the number of moles of sulfuric acid as follows.
No. of moles of
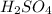 =
=
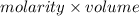
=
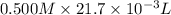
= 0.01085 mol
Now, calculate the moles of KOH as follows.
No. of moles of KOH =
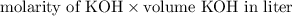
=
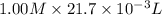
= 0.0217 mol
As the reaction equation is as follows.
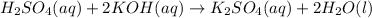
Calculate the number of moles of water as follows.
No. of moles of
 formed =
formed =
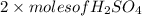 = (moles of KOH)
= (moles of KOH)
= 0.0217 mol
Now, total volume of the solution is as follows.
Total volume of solution = (volume
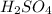 ) + (volume KOH)
) + (volume KOH)
= (21.7 mL) + (21.7 mL)
= 43.4 mL
Total mass of solution =
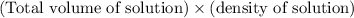
Total mass of solution =
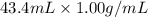
= 43.4 g
Heat change of solution =
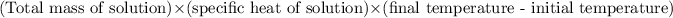
=
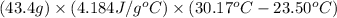
= 1211.18 J
As, heat change of reaction = -(Heat change of solution)
Therefore, heat change of reaction = -1211.18 J
Hence, calculate the change in enthalpy as follows.
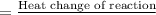
=
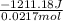
= -55814.56 J/mol
or, = -55.8 kJ/mol (as 1 kJ = 1000 J)
Thus, we can conclude that
 of the given reaction is -55.8 kJ/mol.
of the given reaction is -55.8 kJ/mol.