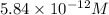Answer : The maximum amount of nickel(II) cyanide is
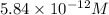
Explanation :
The solubility equilibrium reaction will be:
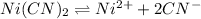
Initial conc. 0.220 0
At eqm. (0.220+s) 2s
The expression for solubility constant for this reaction will be,
![K_(sp)=[Ni^(2+)][CN^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/7xtc6q5t1kgl6gvlwuxxfuqbuddax01fba.png)
Now put all the given values in this expression, we get:
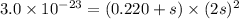
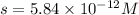
Therefore, the maximum amount of nickel(II) cyanide is