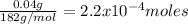Answer:
1) Molecular weight: 18g/mol
Density: at room temperature (25ºC) is 0.997g/mL
Moles: 0.55moles
2) Molecular weight: 182g/mol
Density: at room temperature (25ºC) is 1.11g/mL
Moles:
Step-by-step explanation:
1) The molecular formula of water is
 thus, the molecular weight is the sum of the weights of its atoms.
thus, the molecular weight is the sum of the weights of its atoms.
H: 1g/mol x 2 = 2g/mol
O: 16g/mol x 1 = 16g/mol
 : 2g/mol + 16g/mol = 18g/mol
: 2g/mol + 16g/mol = 18g/mol
The density (δ) of water at room temperature (25ºC) is 0.997g/mL
Therefore, the weight (m) of 1mL of water is:
m = δ.V =
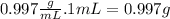
Multiplying the mass by its molecular weight gives the number of moles:
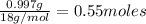
2) The molecular formula of benzophenone is
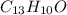 thus, the molecular weight is the sum of the weights of its atoms.
thus, the molecular weight is the sum of the weights of its atoms.
C: 12g/mol x 13 = 156g/mol
H: 1g/mol x 10 = 10g/mol
O: 16g/mol x 1 = 16g/mol
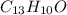 : 156g/mol + 10g/mol + 16g/mol = 182g/mol
: 156g/mol + 10g/mol + 16g/mol = 182g/mol
The density (δ) of water at room temperature (25ºC) is 1.11g/mL
Multiplying the mass by its molecular weight gives the number of moles: