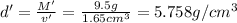Answer:
5.758 is the density of the metal ingot in grams per cubic centimeter.
Step-by-step explanation:
1) Mass of pycnometer = M = 27.60 g
Mass of pycnometer with water ,m= 45.65 g
Density of water at 20 °C = d =
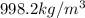
1 kg = 1000 g
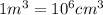
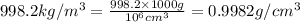
Mass of water ,m'= m - M = 45.65 g - 27.60 g =18.05 g
Volume of pycnometer = Volume of water present in it = V

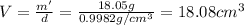
2) Mass of metal , water and pycnometer = 56.83 g
Mass of metal,M' = 9.5 g
Mass of water when metal and water are together ,m''= 56.83 g - M'- M
56.83 g - 9.5 g - 27.60 g = 19.7 g
Volume of water when metal and water are together = v
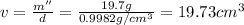
Density of metal = d'
Volume of metal = v' =
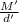
Difference in volume will give volume of metal ingot.
v' = v - V
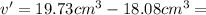
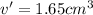
Since volume cannot be in negative .
Density of the metal =d'
=