Answer:
The blood is diluted to 4.9 folds.
Step-by-step explanation:
Steps: 1. Pipette 0.5 mL of blood into 1.0 mL of saline in a fresh test tube.
Initial volume of the Volume of blood before any dilution ,V= 0.5 mL
Volume of saline = 1.0 mL
Volume of the solution after mixing = 0.5 mL + 1.0 mL = 1.5 mL
Step : 2. Remove 0.05 mL of this sample and add to 1.0 mL of the assay reagent in a fresh test tube.
On removal of 0.05 mL from 1.5 mL the volume becomes:
= 1.5 mL - 0.05 mL = 1.45 mL
And 1.0 ml of the essay reagent is added in to 1.45 mL of the solution obtained just above:
Volume of the blood solution = 1.45 mL + 1.0 mL = 2.45 mL
Final volume of the blood solution after dilution = ,V'= 2.45 mL
Fold - dilution =
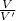
V =Volume of original solution before dilution
V' = Total Volume of solution after dilution
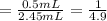
The blood is diluted to 4.9 folds.