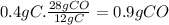Answer:
0.9 g of CO will be produced.
Step-by-step explanation:
A diamond is formed of C atoms. When it is burned, it undergoes a combustion reaction. If the product is CO the combustion is incomplete. The reaction is:
C(s) + 0.5 O₂(g) ⇄ CO(g)
A carat is a unit of weight for diamonds where 1 ct = 200 mg. So, we have 2 ct = 400 mg = 0.4 g of carbon.
In the balanced equation we can see that when 12 grams of C are burned, 28 grams of CO are formed. This is the stoichiometric relationship that we will use to find out the amount of CO formed when 0.4 g of carbon are burned: