Answer:
209.8 kilo Joules heat is required to vaporize 250 g of ethanol at 78.5 °C.
Step-by-step explanation:
Mass of an ethanol = 250 g
Molar mass of ethanol = 46 g/mol
Moles of an ethanol =
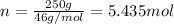
Enthalpy of vaporization of ethanol =
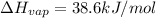
Heat required to vaporize 250 g of ethanol at 78.5 °C : Q

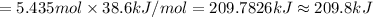
Q = 209.8 kilo Joules