Answer:
2,54x10² mmHg
Step-by-step explanation:
To solve this problem you can use Clausius-Clapeyron equation that serves to estimate vapor pressures or temperatures:
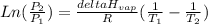
Where:
P1 is 1,00x10² mmHg
ΔHvap is 39,3 kJ/mol
R is gas constant 8,314x10⁻³ kJmol⁻¹K⁻¹
T1 is 34,90°C + 273,15 = 308,05 K
T2 is 54,81°C + 273,15 = 327,96 K
Thus:
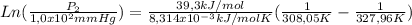
Thus, P2 is 2,54x10² mmHg
I hope it helps!