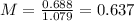Answer:
Step-by-step explanation:
For this question, we have to assume that we have 1 L of solvent. With the molality equation we can calculate the moles of solute, so:
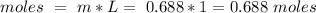
Then we can calculate the grams of solute using the molar mass :
molar mass of
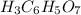 =192.12 g/mol
=192.12 g/mol
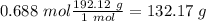
The density for water is 1 Kg/L, therefore 1 L of water equal to 1 Kg, the total mass of the solution would be:
1000 g + 132.17 g = 1132.17 g
With this value we can calculate the volume of the solution using the density, so:
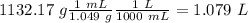
Finally, we can calculate the molarity diving the moles by the volume, so: