Answer: The partial pressure of carbon dioxide having solubility 0.886g/100mL is 4182.4 mmHg
Step-by-step explanation:
Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the liquid.
The equation given by Henry's law is:
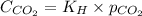 ......(1)
......(1)
where,
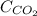 = solubility of carbon dioxide in water = 0.161 g/100 mL
= solubility of carbon dioxide in water = 0.161 g/100 mL
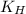 = Henry's constant = ?
= Henry's constant = ?
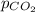 = partial pressure of carbon dioxide = 760 mmHg
= partial pressure of carbon dioxide = 760 mmHg
Putting values in equation 1, we get:
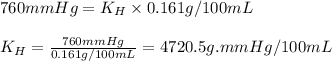
Now, calculating the pressure of carbon dioxide using equation 1, we get:
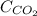 = solubility of carbon dioxide in water = 0.886 g/100 mL
= solubility of carbon dioxide in water = 0.886 g/100 mL
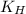 = Henry's constant = 4720.5 g.mmHg/100 mL
= Henry's constant = 4720.5 g.mmHg/100 mL
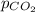 = partial pressure of carbon dioxide = ?
= partial pressure of carbon dioxide = ?
Putting values in equation 1, we get:
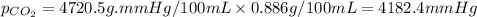
Hence, the partial pressure of carbon dioxide having solubility 0.886g/100mL is 4182.4 mmHg