Step-by-step explanation:
(w/w) % : The percentage mass or fraction of mass of the of solute present in total mass of the solution.
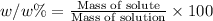
1) 100 g of 0.500% (w/w) NaI
Mass of solution = 100 g
Mass of solute = x
Required w/w % of solution = 0.500%
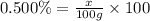
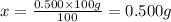
0.500 grams of solute needed to make 100 g of 0.500% (w/w) NaI.
2) 250 g of 0.500% (w/w) NaBr
Mass of solution = 250 g
Mass of solute = x
Required w/w % of solution = 0.500%
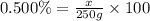
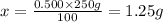
1.25 grams of solute needed to make 250 g of 0.500% (w/w) NaBr
3) 500 g of 1.25% (w/w) glucose
Mass of solution = 500 g
Mass of solute = x
Required w/w % of solution = 1.25%
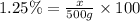
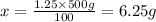
6.25 grams of solute needed to make 500 g of 1.25% (w/w) (glucose)
4) 750 g of 2.00% (w/w) sulfuric acid.
Mass of solution = 750 g
Mass of solute = x
Required w/w % of solution = 2.00%
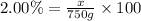
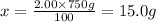
15.0 grams of solute needed to make 750 g of 2.00% (w/w) sulfuric acid.