Answer: The volume of water required is 1.65 mL and the volume of acetic anhydride required is 0.65 mL
Step-by-step explanation:
To calculate the number of moles, we use the equation:
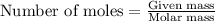 .....(1)
.....(1)
Given mass of p-aminophenol = 0.50 g
Molar mass of p-aminophenol = 109.14 g/mol
Putting values in equation 1, we get:
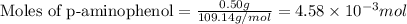
To calculate volume of solution, we use the equation:
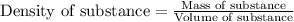 ......(2)
......(2)
It is given that 20 molar equivalent so p-aminophenol is added:
Moles of water =
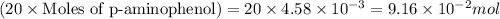
Mass of water is calculated by using equation 1, we get:
Molar mass of water = 18 g/mol
Mass of water =
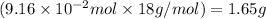
Now, calculating the volume of water by using equation 2, we get:
Density of water = 1 g/mL
Mass of water = 1.65 g
Putting values in equation 2, we get:
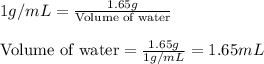
Hence, the volume of water required is 1.65 mL
It is given that 1.5 molar equivalent so p-aminophenol is added:
Moles of acetic anhydride =
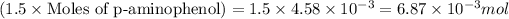
Mass of acetic anhydride is calculated by using equation 1, we get:
Molar mass of acetic anhydride = 102.1 g/mol
Mass of acetic anhydride =
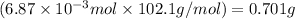
Now, calculating the volume of acetic anhydride by using equation 2, we get:
Density of acetic anhydride = 1.08 g/mL
Mass of acetic anhydride = 0.701 g
Putting values in equation 2, we get:
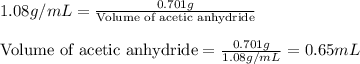
Hence, the volume of acetic anhydride required is 0.65 mL