Answer:
Molecular mass of X is 73g/mol
Step-by-step explanation:
To answer this question you need to know that Freezing-point depression (A colligative property) is the decrease of the freezing point of a solvent on the addition of a non-volatile solute. The formula is:
ΔT = Kf m
Where ΔT is the freezing point depression (2,57°C - (-1,4°C) = 3,97°C Where 2,57°C is the melting point of formamide.
Kf that is freezing point molar constant of the solvent (4,25°C/m)
And m that is molality (moles of solute/kg of solvent). Replacing:
m =
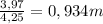
Knowing you have 80,0g of solvent ≡ 0,0800 kg:
0,934 mol solute/kg solvent×0,08 kg solvent = 0,07472 moles of solute ≡ moles X
As grams of X are 5,42, molecular mass of X is:
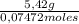 = 73 g/mol
= 73 g/mol
I hope it helps!